Published on January 10, 2024 by Amit Kumar
Introduction
Cardiovascular disease is one of the world’s leading causes of mortality and morbidity, causing 17.9m deaths a year, according to the World Health Organization. Cardiovascular disease refers to problems affecting the heart and blood vessels, some of the most common being atherosclerosis, stroke, heart failure and arrhythmia. Causes of cardiovascular disease can vary depending on the specific type of disease, but main risk factors include high blood pressure, high cholesterol, smoking, diabetes, obesity and an unhealthy diet. Researchers have discovered a number of drug targets from animal studies and evaluated them in the treatment of heart disease in recent years; these have provided exciting suggestions for new therapies and biomarkers for disease diagnosis. However, only a few new agents have been clinically approved, and current treatment methods are lagging. Medical science has developed a number of treatments and diagnostics for cardiovascular disease, but modern technologies need to be integrated with the treatment to improve patient outcomes and help the cardiac devices sector grow.
A growing market
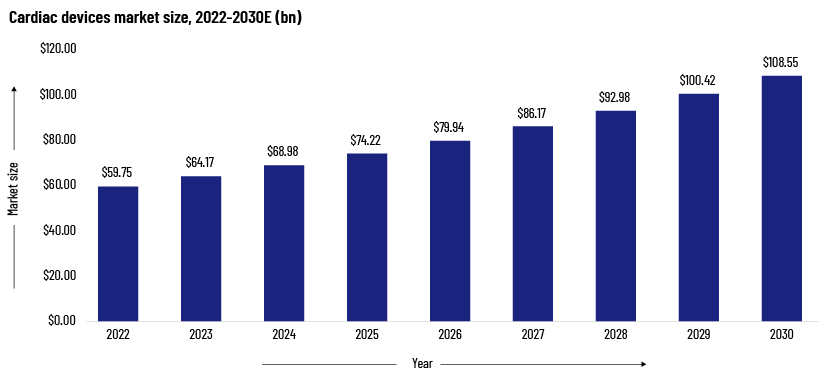
The global cardiac devices market was valued at USD59.75bn in 2022 and is expected to grow to more than USD108.55bn by 2030, at a CAGR of 7.9%. The high prevalence of cardiovascular disease is expected to increase demand for novel treatment options.
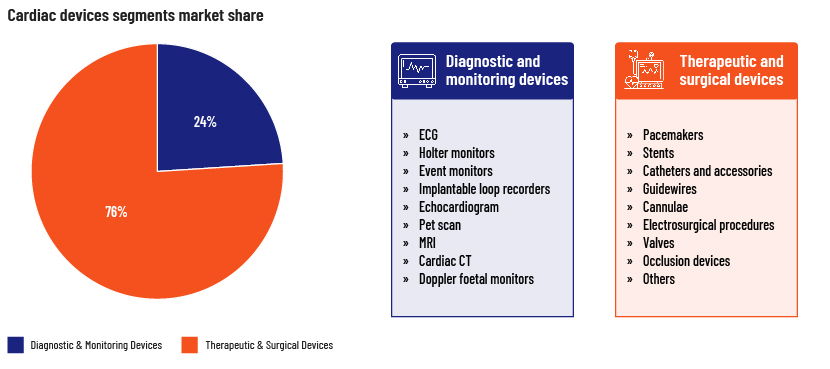
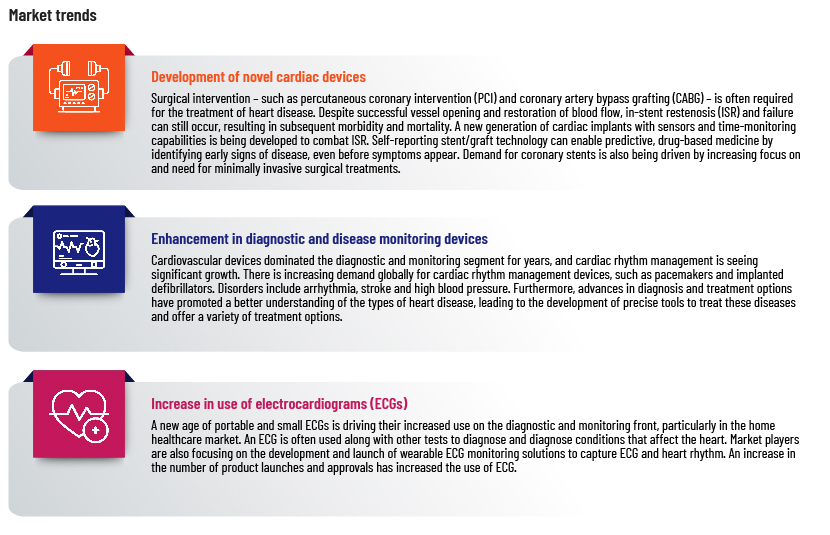
Cardiac devices market segmentation
-
Therapeutic and surgical devices
The therapeutics and surgical devices segment dominates the global market, with a revenue share of 76% in 2022, driven by increasing awareness of cardiovascular disease and the ease of use of such devices. Demand has increased particularly for cardiac rhythm management devices, stents and catheters.
-
Diagnostics and monitoring devices
These are used in ECG monitors, Holter monitors and other imaging solutions to make it easier to diagnose certain heart conditions, such as irregular heartbeat and coronary artery disease. Pharmaceutical and healthcare companies are working with researchers to identify new biomarkers and develop devices that are more accurate and that can help in early diagnosis of cardiovascular disease. The increased use of wearable technologies for continued monitoring and tracking also drives growth of diagnostic and monitoring devices.
Types of cardiovascular medical devices
-
Automated external defibrillators (AEDs): These assist in restoring regular coronary heart rhythm in patients who have issues with pumping blood, also known as cardiac arrest. AEDs examine coronary heart rhythm and could help physicians decide whether shock treatment is needed to repair an irregular heartbeat.
-
Cardiac ablation catheters: These are long, flexible tubes that are threaded into or onto the heart to deal with abnormal heartbeats. They modify the small regions of coronary heart tissue that inflict abnormal coronary heart rhythms.
-
Cardiovascular angioplasty devices: These are long, thin, flexible tubes that are threaded right into a coronary heart or different blood vessel to open narrowed or blocked areas. They are meant to enhance blood flow to the coronary heart, reduce chest pain and deal with coronary heart attacks.
-
Cardiac pacemakers: These are powered by small batteries and implanted in the body. They are used when the coronary heart beats too slowly, when the heart’s electric impulses need to be read or when electric stimulation is needed to make the heart beat at a more suitable rate.
-
Implantable cardioverter defibrillators (ICDs): These monitor coronary heart rhythms and supply shocks if abnormally rapid rhythms are detected. They record the coronary heart’s electric patterns so doctors can check them.
-
Prosthetic (artificial) heart valves: These are used to replace unhealthy heart valves and are available in two different types: mechanical valves are made of man-made materials, and "bioprosthetic" valves are made from animal tissue.
-
Stents: These are small, lattice-shaped, steel tubes that are inserted completely into an artery to improve blood flow. Some have capsules that can clear blocked arteries.
-
Ventricular assist devices (VADs): These are mechanical pumps that help hearts pump blood effectively. They are authorised for use for short periods of time, until the heart regains function. Some are authorised for use for long-term relief for patients with excessive coronary heart failure.
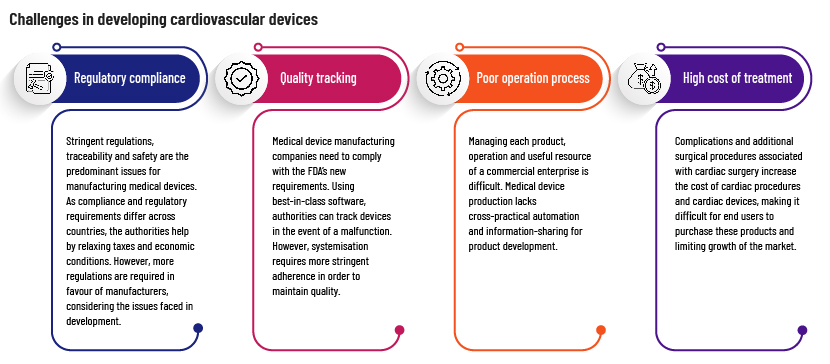
Challenges in developing cardiac devices
Recent large approvals and market entry of novel cardiac devices
1. Abbott receives US Food and Drug Administration (FDA) approval for the world's first dual-chamber leadless pacemaker
In July 2023, the FDA approved Abbott’s AVEIR dual-chamber (DR) leadless pacemaker system, the world’s first, two-chamber pacing system that treats individuals with irregular or slow heart rhythms. With Abbott's proprietary i2i communication technology, the AVEIR device delivers synchronised or controlled cardiac output between two ventilators based on the individual's clinical needs. The i2i technology uses high-frequency pulses to transmit information through the characteristics of the body's blood flow between the individual and each wireless device. Each implant uses an integrated device that communicates beat-to-beat. Conductive communication is important because it uses less battery current than inductive, radio frequency or Bluetooth communication. Shorter, smaller and thinner than an AAA battery, the AVEIR pacemaker is implanted through a minimally invasive process. The device is attached to the top of the heart using a screw system called a helix, which enables the device to be retrieved if replacement is required.
2. New implantable cardiac monitor with artificial intelligence
In June 2023, Biotronik announced the world’s first implantation of its BIOMONITOR IV implantable cardiac monitor (ICM). It combines Biotronik’s smart ECG technology with artificial intelligence to reduce false diagnoses by 86% and preserve 98% of true episodes. It is also the only ICM that can distinguish between premature atrial contractions (PACs) and premature ventricular contractions (PVCs), providing healthcare professionals with a better risk assessment tool. In addition to artificial intelligence, BIOMONITOR IV has many other benefits for patients and healthcare professionals. Long-lasting batteries and wireless connectivity enable seamless data transmission and monitoring, reducing the need for frequent in-person hospital visits. Biotronik also offers electronic health record (EHR) data-entry capabilities that enhance clinical workflow.
3. Medtronic’s small cardiac devices Micra AV2 and Micra VR2 receive FDA approval
In May 2023, Medtronic announced that it had received FDA approval for the Micra AV2 and Micra VR2 – the next generation of small pacemakers with wireless technology. These devices offer longer battery life and easier programming than the previous Micra pacemakers, in addition to the many advantages of air stimulation. With around 40% longer battery life than previous generations of devices, the average battery life of the Micra AV2 and Micra VR2 is almost 16 and 17 years, respectively. This means that the more than 80% of patients receiving Micra will need only one device in their lifetime. Like a multivitamin, a Micra pacemaker is one-tenth the size of a traditional pacemaker. Unlike traditional pacemakers, Micra pacemakers do not require a surgical incision or "pocket" under the skin, eliminating potential complications from lead and “pockets”.
Outlook for cardiac medical devices
-
Home-based diagnosis and care
The Centers for Medicare & Medicaid Services (CMS) say that spending on home care will increase to USD201bn by 2028. The market is seeing a growing shift in care – from the hospital to the home. Medical products and cardiac devices must, therefore, be designed to fit the environment. Cardiac devices used for diagnosis at home would be accepted if they have features that suit this environment.
-
Wearable trackers and technologies
With increasing indications and global access to cardiac rhythm management (CRM) and cardiac implantable electronic devices (CIEDs), the number of patients requiring CIED monitoring is increasing. In addition, technological innovations have led to the emergence of magnetic resonance imaging (MRI) as an effective method for facility-based patient care. The use of wearable technology to monitor health and their potential applications in cardiology have become a topic of discussion in recent years. Smart devices not only help monitor physical activity, but also provide personalised nutrition advice, improve sleep quality and recommend medication based on heart rhythm.
-
Remote monitoring and big data analytics
Remote monitoring helps hospitals move data beyond patients, enabling early detection of critical illnesses. Smaller devices, more data and connected healthcare lead to better patient outcomes. Using tools such as data analytics, artificial intelligence and predictive models, healthcare companies are streamlining care to make it more effective, accessible and equitable. Data is the key to creating timely treatment and positive patient outcomes.
-
Artificial intelligence (AI)
Using tools such as AI and machine learning, healthcare professionals can gain new insights on the human body to create solutions that help patients and doctors. With advances in AI and data analysis, medical devices and cardiac devices are improving patient care by helping doctors plan medications like never before.
Conclusion
The future of cardiac devices is bright, but integration and adoption of new technologies remain a challenge. Increasing incidence of cardiovascular disorders has resulted in an unmet need for treatment options that are safe and effective for longer. An individualised approach and personalised risk planning using research are needed to identify new strategies and technologies that can help diagnose and treat patients. New solutions could replace traditional methods and unnecessary procedures in treating and diagnosing patients at risk of cardiovascular disease. In addition, with increased demand for remote monitoring and home-based treatment solutions, the regulatory landscape needs to be updated in terms of ethical and data-security issues. Healthcare companies are constantly striving to find new treatment solutions for every aspect of cardiovascular disease using modern technology. AI and data analytics would significantly impact the development of devices and diagnostics that are least invasive and can work for longer.
Sources:
-
Cardiovascular Devices Market Size, Share, Report 2023 to 2032 (precedenceresearch.com)
-
Cardiovascular Devices Market Size, Share & Growth (mordorintelligence.com)
-
Cardiovascular Devices Market Share | Industry Outlook, 2030 (psmarketresearch.com)
-
Emerging Trends in Cardiovascular Devices – MedTech Intelligence
-
The Future of Cardiac Rhythm Management Device Technology | DAIC (dicardiology.com)
-
How Home Health Will Evolve in the Year Ahead (healthpayerintelligence.com)
What's your view?
About the Author
Amit Kumar has more than five years of experience in pharma and healthcare consulting and advisory services. As a member of the Life Sciences Consulting Team at Acuity Knowledge Partners (Acuity), he provides healthcare corporate and consulting clients with scientific, clinical and commercial insights. He is proficient in primary-/secondary-market research, competitive intelligence, market assessment, opportunity assessment, therapy-area assessment, clinical trial analysis and scientific conference coverage.
Prior joining Acuity, Amit Kumar worked for Johnson & Johnson and ZS Associates, where he was responsible for ICSR management and secondary-market research, respectively.
Like the way we think?
Next time we post something new, we'll send it to your inbox