Published on May 23, 2024 by Dilpreet Kaur
AI in healthcare: accelerating breakthroughs
AI in healthcare refers to a group of technologies that enable machines to sense, understand, act and learn to carry out clinical and administrative healthcare tasks. Today's AI can enhance human behaviour, in contrast to legacy technologies that are merely algorithms or tools to support humans.
AI and machine-learning (ML) tools are changing the way clinical development is carried out, offering significant time and cost savings while providing better and faster insights to support decision-making.
The success rate of drug candidates that complete clinical trials and receive marketing approval has stayed mostly stable at 10-20% over the past few decades. A number of factors contribute to failure at clinical-trial stage; these include inadequate study design, insufficient patient recruitment, incorrect subject categorisation and high rates of attrition of participants in clinical trials. To overcome these obstacles and expedite the clinical-trial process, stakeholders in the pharmaceutical sector are looking for creative ideas and strategies, one of which involves combining AI in drug development. The different types of AI technologies essential in the healthcare sector are ML, the internet of things (IoT), the internet of medical things, robotic process automation, rule-based expert system and natural language processing (NLP).
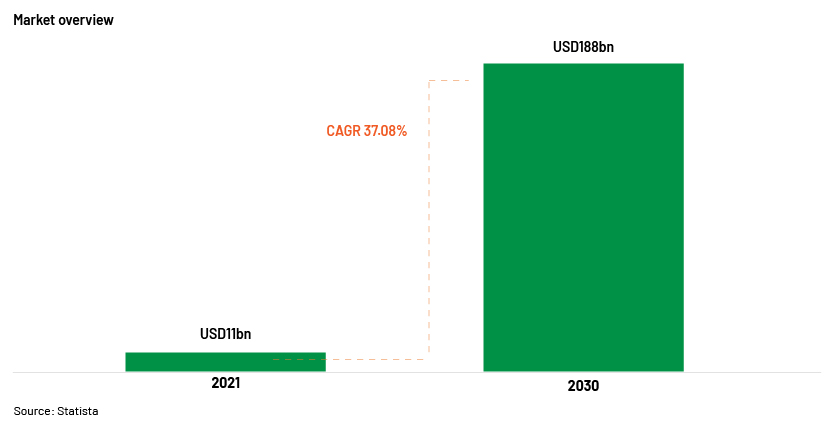 Source: AI in healthcare market size worldwide 2030 | Statista
Source: AI in healthcare market size worldwide 2030 | Statista
The global AI market in healthcare was valued at USD11bn in 2021, according to ,it is forecast to reach USD188bn by 2030.
Substantial investment in the field indicates growing interest in AI in clinical trials. The UK government invested USD17m in AI research in March 2023 for cancer checks, rare-disease diagnosis, identifying women at high risk for preterm labour or birth and improving overall speed and accuracy of diagnosis. Similarly, the National Institutes of Health (NIH) announced in September 2022 a USD130m investment over four years to enhance AI use in biomedical and behavioural research. This inflow of investment indicates healthy growth potential for AI in the clinical-trials market over the forecast period.
More than 130 companies currently specialise in offering different AI software and services to expedite clinical research. Eighty percent of them concentrate on using deep-learning and ML algorithms to access several data points at once and reduce data-based errors. To increase their capacities and develop extensive product/service portfolios, stakeholders have been collaborating and developing partnerships with other sector and non-sector entities on AI in clinical trials. Key players in this segment include Saama Technologies, Antidote and Deep 6 AI.
Key drivers
The main driver of this spike is the pressing need to control rapidly increasing expenses associated with clinical trials. When combined, key clinical health AI applications could result in annual savings of USD150bn for the US healthcare economy alone by 2026, according to Accenture analytics.
-
By using large-scale healthcare datasets and AI, ML and NLP tools, companies could evaluate and choose the best primary and secondary endpoints for research designs, ensuring that the most pertinent protocols are developed for payers, regulators and patients.
-
Finding sites with the best potential for recruitment and recommending effective recruitment tactics are two ways in which AI and ML could help reduce risks such as the possibility of failure, long timeframes and increased expenses.
-
By assessing risk and using predictive modelling based on previous data, generative AI can expedite and simplify the planning process. Generative AI is able to recognise patterns and relationships and highlight past hazards and adverse outcomes by examining data from prior clinical trials.
-
ML algorithms such as generative adversarial networks (GANs) and recurrent neural networks (RNNs) are revolutionising the speed and ability with which potential drug candidates are identified and can even facilitate new drug designs.
-
Generative AI produces thorough, precise clinical study reports (CSRs) more quickly and with a lower chance of error.
-
An AI-powered platform can monitor any kind of input, including patient data, site performance metrics and data from other sources. It can also conduct routine checks to ensure that processes meet industry requirements.
Regional analysis
North America holds the largest market share in this space due to large R&D investment, rapid technological progress, growing adoption of advanced solutions, the presence of foreign rivals and the pursuit of new patents. Businesses in the region also have access to more capital and expansion options, likely to encourage market expansion.
Germany and the UK have sizeable patient populations and well-developed medical knowledge. Doctors support clinical trials because they increase patient recruitment, which is advantageous for patients and research. Acceptance of patient-friendly and technologically advanced clinical-trial solutions has expanded in Europe due to the medical community's support and acceptance of these solutions. Furthermore, legal aspects are still developing in Germany, although they are fully matured in France and the UK. Because of increased funding and protocols, clinical trials are likely to be more successful in Europe.
Key developments
In October 2023, Unilearn.AI, which built an ML platform that creates “digital twin” profiles of patients in clinical trials, had raised USD15m to expand partnerships and accelerate regulatory approval.
In July 2023, Insilico Medicine announced the initiation of the phase II clinical trial of INSO18_055 for the treatment of idiopathic pulmonary fibrosis. INSO18_055 is the first drug with a novel AI-discovered target and a novel AI-generated design.
In January 2023, Eligo Health Research, a healthcare-enabling research organisation, introduced DataAI Connect, a new data and technology platform that will enable rapid, data-driven clinical research. A scalable, end-to-end data platform, DataAI Connect creates efficiencies across the clinical study workflow, reducing human error and rapidly digesting patient data to accelerate clinical trials.
How market participants view AI
“The time frame for developing a new drug from ground zero takes about 10 to 12 years, using USD1.2bn in a very conservative estimate. With AI, you can shorten it easily by more than half, to about 5 years. That’s what we are seeing today. A lot of people in drug development will agree with that, especially for drug discovery to get the right drug and go to the next stage.”
--Gideon Ho, Co-Founder and CEO, HistoIndex (07/21/2023)
“Life science companies face significant headwinds in terms of the complexity and cost of conducting clinical research and bringing new treatments to market. Saama’s unified platform demonstrates to trial sponsors and CROs the power of automation to drive efficiencies as well as enable greater visibility into clinical data to deliver novel treatments to patients.”
--Vivek Sharma, CEO, Saama Technologies (06/20/2023)
Outlook
AI is integrating itself into different stages of the drug-development process, serving as a diagnostic tool to support the process and helping clinicians make better judgements.
AI would also have the potential to be used in other areas such as analysing medical photos, X-rays and scans; diagnosing medical issues; and developing treatment plans. AI applications (and, probably, applications not yet thought of) would continue to help streamline a variety of tasks, from answering phones to analysing trends in population health.
The hype around AI is also likely to trigger investment in the biotech sector after a difficult economic period, when companies struggled to raise capital.
How Acuity Knowledge Partners can help
-
Our life sciences and solutions and pharmaceutical research help manage and analyse large volumes of clinical-trial data, including patient records and genomic data.
-
Analyse historical trial data to predict and optimise patient enrolment strategies
-
Assist in ensuring that AI applications in clinical trials adhere to regulatory standards and compliance requirements
-
-
Our bespoke investment banking analytics offer detailed transaction support and in-depth research, financial analysis and presentation skills.
-
These analytics help identify trends, assess market potential and optimise investment portfolios, enabling clients to allocate resources more efficiently and make informed decisions in AI-driven clinical trials.
-
Sources:
-
How AI is shaping clinical research and trials (labiotech.eu)
-
BDCC | Free Full-Text | Artificial Intelligence in Pharmaceutical and Healthcare Research (mdpi.com)
-
Top 10 Companies Supporting Use of AI in Clinical Trials (rootsanalysis.com)
-
First Generative AI Drug Begins Phase II Trials with Patients | Insilico Medicine
-
Artificial Intelligence in Healthcare Market Size, Report 2022-2030 (precedenceresearch.com)
-
Life Sciences Solutions and Pharmaceutical Research | Acuity Knowledge Partners (acuitykp.com)
-
Investment Banking Research and Analytics | Acuity Knowledge Partners (acuitykp.com)
-
AI in Clinical Trials Market Size & Share | Global Report, 2035 (rootsanalysis.com)
Tags:
What's your view?
About the Author
Dilpreet joined Acuity Knowledge Partners’ investment banking team in 2022, having an overall experience of 4+ years across industries. She has worked in different Healthcare and Life Sciences firms serving different position from being a Business Analyst to Project Manager for a variety of leading healthcare clients. She has gained exposure in preparing industry reports, company profiles, in-depth industry insights, case studies, and company focused discussion documents related to various industries at Acuity.
Like the way we think?
Next time we post something new, we'll send it to your inbox