Published on June 11, 2020 by Raghu Patale
It is more than five months since the first cases of COVID-19 were detected in Wuhan, China, and the whole world has slowly adjusted to the new normal amid the pandemic. The global crisis has necessitated a 360-degree shift in our lives and a significant change in the way many sectors operate, especially healthcare. This sector has struggled with attending overwhelmingly large patients and identifying therapeutic intervention for COVID-19 and has been one of the most affected, while also probably benefiting the most. Due to this shift in focus amid the pandemic, the different segments of the pharma value chain have been affected differently. The following chart summarises the effects and indicate if any steps are taken to ease them.
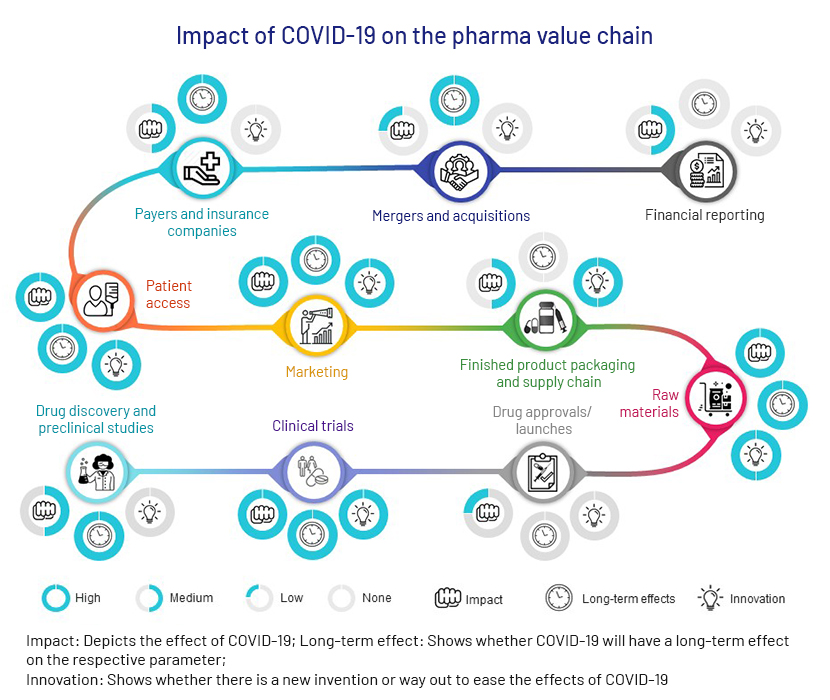
1. Drug discovery and preclinical studies
The whole world is investing in and focusing energies on discovering treatments for COVID-19. There are as many as 200 drug and vaccine candidates under development as of 18 May 2020, with more than 80% in the discovery and preclinical phase. Research activity has shifted completely to finding a cure for this virus. The non-COVID-19-related pipeline would, therefore, be affected for the coming few years.
2. Clinical trials
The pandemic has already halted 1,300+ US-based clinical trials, making it one of the worst affected segments. Of these, 300+ interventional studies were sponsored by industries. Suspending them means a delay in the therapies reaching the market. On the contrary, there are 1,800+ ongoing COVID-19-related trials indicating huge focus on the pandemic; this 1800+ trials includes observational and interventional studies funded by universities, governments and industries.
The regulatory authorities had started taking steps to digitalise clinical trials even before this pandemic began, but the matter has now become more urgent. Sponsors and the regulatory agencies are looking for innovative options such as telemedicine, videoconferencing, mailing trial drugs, dispatching healthcare workers for sample collection and protocol changes.
Most companies have paused patient enrolment, except Pfizer, which has announced it will resume clinical trials. The UK National Institute for Health Research (NIHR) also released a framework recently to restart paused clinical trials.
3. Drug approvals/launches
The pandemic has had literally no effect on FDA review and approval systems; in fact, the number of approvals has increased by more than 50% this year from last year. The impact on 1Q drug launches was also minimal; only Bristol-Myers Squibb and Acacia Pharma considered postponing their launches.
The pandemic is likely to have the largest effect on drug launches planned for 2Q, and a moderate effect on those planned for 3Q. Drug launches are likely to normalise by 4Q 2020.
4. Raw materials
Pharma companies’ dependence on Asian countries for active pharmaceutical ingredients (APIs), intermediates and reagents has led to disruption in the raw material supply chain. The US sources 80% of its APIs from overseas, a substantial portion from Asia, while Europe imports more than 50% of its APIs from India and China. Because of this dependence, generic drug manufacturers are affected severely, whereas innovators are less affected, as they manufacture their APIs elsewhere.
Mobius Capital Partners forecasts that companies around the world will alter their supply chains so as to be less dependent on China. France and Ireland are already planning to diversify their supply chains. Economists and analysts expect globalisation to be reversed after the pandemic and regional supply chains to be created.Sanofi has capitalised on this opportunity by spinning off its six manufacturing facilities with 3,100 employees, planning to become the second-largest API manufacturer in the world by 2022.
On the strategic front, the pandemic has made US officials concerned about China’s control of the US drug supply; the US could implement new policies to promote local manufacturing.
5. Finished products, packaging and supply chains
Drugs are categorised as essential goods; hence, there is support from local governments to restore the manufacture of APIs and finished products. For example, pharma company employees are allowed to work with minimal restrictions, as is the case in India. However, with logistics and distribution services all but halted, the industry is experiencing decreased production capacity that would eventually affect the availability of medicines. We also expect stricter inspections if guidelines such as those on hygiene, social distancing, cleaning and disinfection are to be closely followed.
Consulting firms such as McKinsey and LogisticsIQ envisage the digitalisation of the supply chain or a transformation to the next generation of supply chains. Next-generation supply chains digitise end-to-end performance management using a combination of 5G, IoT, AI, drones and robots.
6. Marketing
Sales teams are the biggest game changers for pharmaceutical markets, but most companies have suspended their work, as they are susceptible to infection when visiting doctors. More companies are adopting newer sales channels such as email, virtual marketing, and digital communication with physicians. To compensate the need for face-to-face interaction between sales personnel and physicians, some have come up with innovative messaging services or personalised content to reach customers and physicians directly. Given benefits such as longer interactions and interaction at a time convenient to them, physicians may prefer to continue to engage via digital channels in the future as well, or choose a hybrid model of communication.
7. Patient access
Fearing infection, patients are not actively renewing prescriptions. Due to uncontrolled spread of infection, there is a strong inclination towards digitalising healthcare systems, paving the way for services such as remote patient monitoring and telemedicine. All non-urgent consultations are currently moderated by telehealth and remote health monitoring. The US government is supporting this, providing USD200m in funding to support telehealth programmes (through the US Federal Communications Commission) and also allowing doctors and medical professionals to Practice across state lines. Remote monitoring, telehealth platforms, AI-powered health assessment apps and technologies have gained momentum lately, but the pandemic has ensured that these technologies are essential and here to stay.
8. Payers and insurance companies
Payers (companies that pay for administered medical services, such as insurance companies) and regulatory authorities were reluctant until 2019 to approve invoices for digital patient monitoring. With routine healthcare services now available through telehealth, CMS is promoting this by waiving all co-payments and deductibles for telehealth services.Insurance majors such as United Healthgroup, Anthem and Aetna are leading this drive by waiving co-payments and deductibles.
Apart from the pharma value chain, the pandemic has had a considerable effect on pharma mergers and acquisitions and revenue:
9. Mergers and acquisitions (M&A)
The pandemic will have an impact on the transactional activity, at least in the short term for both buyers and sellers. According to a Kaufman Hall report, 29 transactions were announced in 1Q 2020, which is consistent with previous years. Considering the ongoing disruptions, 1Q performance cannot be considered when predicting activity for the remainder of 2020. M&A activity also seems to have tapered off after 12 March.
Due to robust demand with relatively minimal supply disruptions and attractive valuations even in tough times, deal activity in the healthcare sector may accelerate. The pandemic is likely to be a catalyst for more M&A when it eases, especially acquisitions of digital technology companies and financially stricken organisations. Repositioning through M&A may also reduce the impact of future pandemics and competition.
10.Financials
Financials of pharma companies show mixed trends, with many companies reporting increases in 1Q revenue amid the crisis. Of the pharma giants, Merck, Eli Lilly and AstraZeneca saw their revenue growing by more than 10%. Industry experts believe these results are momentary and were due to stockpiling, and should normalise by the end of the year. In contrast, companies working in niche segments such as orthopaedics, ophthalmology, and aesthetic surgery, driven primarily by physician consultations, are the worst hit due to social-distancing measures.
What’s next?
Unlike many industries struggling to identify a suitable response to the COVID-19 impact, the healthcare sector has infrastructure in place, with technologies such as remote patient monitoring, automated manufacturing facilities, and AI in drug discovery. The pandemic has resulted in very heavy lifting by healthcare workers, but the sector as a whole is somewhat immune to effects of a downturn. The crisis has largely acted as catalyst for transforming the sector, making it more sustainable and better prepared for future exigencies.
As Raymond Nomizu said, one thing is for sure: “When we look back five years from now, I fully expect to see a drop in the number of breakthrough new medications being approved – a drop you can directly tie to this crisis”.
About Acuity Knowledge Partners
We at Acuity Knowledge Partners have a deep understanding of industry dynamics and nearly two decades of experience that enable us to empower and assist healthcare companies navigate through these tough times by strengthening their strategies.
To help our clients navigate both the people and business impact of COVID-19, we have created a dedicated hub containing a variety of topics including our latest thinking, thought leadership content and action oriented guides and best practices.
What's your view?
About the Author
Raghu Patale serves as Delivery Manager leading the Life Sciences Corporate Strategy Research and Consulting vertical. His responsibilities include thought leadership, setting up new client engagements, client management, and generating business insights. He has over 10 years of experience in conducting life science research as a competitive intelligence and strategy consultant. He has supported a wide spectrum of client engagements focusing on competitive intelligence, therapy area research, market opportunity assessments, M&A support and report writing in oncology and other therapy areas for US, EU5, and Asian geographies.
Prior to this, Raghu was a Group Manager, leading the Pharma Practice at Evalueserve. He holds a Master’s degree in Pharmaceutical..Show More
Like the way we think?
Next time we post something new, we'll send it to your inbox